Have you ever picked up a prescription and noticed the pill looks different-maybe a different color or shape-but the name on the bottle is the same as your usual brand? You might’ve thought, "Is this the real thing?" The answer is yes. It’s not a knockoff. It’s an authorized generic.
Authorized generics are the exact same drug as the brand-name version you know. Same active ingredient. Same dose. Same shape. Same way your body absorbs it. The only difference? The label doesn’t have the brand name on it. No logo. No fancy packaging. Just the generic name and a manufacturer’s code. It’s like buying the same car but without the badge on the hood.
How Authorized Generics Are Made
Most people think generic drugs are made by separate companies that copy the brand-name drug. That’s true for traditional generics. But authorized generics are different. They’re made by the very same company that makes the brand-name drug.
For example, Pfizer makes Lipitor, the brand-name cholesterol drug. When the patent expired, Pfizer didn’t just sit back and let other companies jump in. Instead, they created Greenstone-a subsidiary that makes and sells Lipitor under a generic label. Same factory. Same equipment. Same quality control. Same pills. Just a different box.
This isn’t a loophole. It’s written into U.S. law. The FDA calls it a "listed drug" under the original brand’s New Drug Application (NDA). That means no extra testing is needed. No bioequivalence studies. No long approval process. The drug was already approved. All the company has to do is notify the FDA they’re selling it under a different label.
Why This Matters
Here’s the twist: authorized generics compete with traditional generics. When a brand-name drug’s patent expires, the first company to file for a generic version gets 180 days of exclusive rights to sell it. That’s supposed to encourage competition and lower prices.
But if the brand company launches its own authorized generic during that 180-day window, it can undercut the first generic maker. Suddenly, the company that spent millions to challenge the patent is facing competition from the original maker-selling the exact same product at the same price.
This has caused real tension in the industry. Critics say it undermines the whole point of the Hatch-Waxman Act, which was designed to get generics to market quickly. Supporters argue it gives consumers more choices and keeps prices low.
Authorized Generic vs. Traditional Generic vs. Brand Name
Let’s break it down simply:
| Feature | Brand Name | Traditional Generic | Authorized Generic |
|---|---|---|---|
| Who makes it? | Original drug company | Separate generic manufacturer | Original drug company |
| Active ingredient | Identical | Identical | Identical |
| Inactive ingredients | Brand-specific | May differ | Identical to brand |
| Regulatory path | New Drug Application (NDA) | Abbreviated NDA (ANDA) | Uses brand’s NDA |
| Appears in FDA Orange Book? | Yes | Yes | No |
| Price compared to brand | Full price | Lower | Same as traditional generic |
That last row is key. Authorized generics aren’t cheaper than traditional generics. They’re priced the same. But because they’re made by the brand company, they’re often more reliable in terms of consistency. No surprises. No changes in how the pill works.
What You’ll See on the Label
If you get an authorized generic, you might notice:
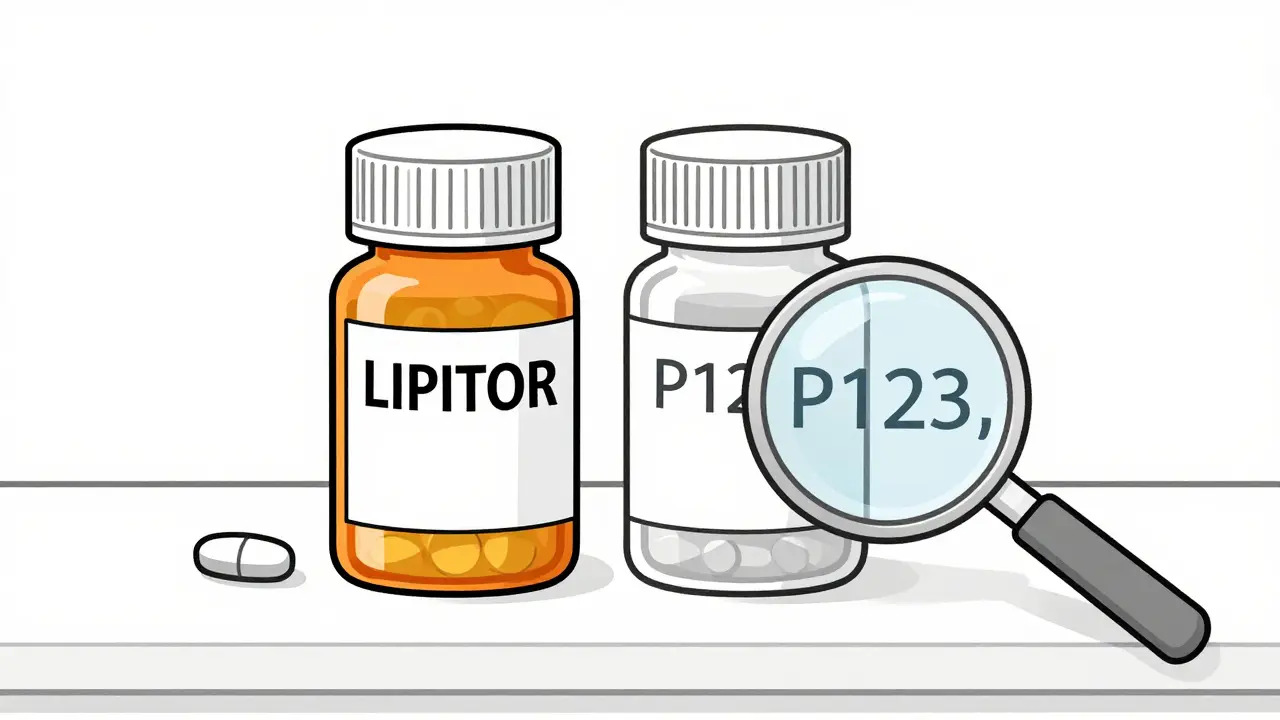
- The pill looks slightly different-maybe a different color or marking.
- The name on the bottle is the chemical name, not the brand name.
- The manufacturer is a company you’ve never heard of, like Prasco or Patriot.
These changes are just to avoid trademark confusion. The FDA doesn’t allow the same packaging as the brand. So, the pill might be blue instead of yellow, or have a "P123" imprint instead of "LIPITOR." But if you check the active ingredient, it’ll match exactly.
Some patients get confused. They think, "This isn’t the same as what I used to take." But it is. A 2023 study from Health Affairs found that patients who switched from brand to authorized generic reported no change in effectiveness or side effects.
Why Pharmacists Get Confused
Here’s a hidden problem: authorized generics don’t show up in the FDA’s Orange Book. That’s the go-to reference pharmacists use to check if a generic is interchangeable with a brand.
So when a prescription comes in for "atorvastatin," and the pharmacist sees an authorized generic on the shelf, they can’t just pull it up in the system. They have to check a separate FDA list, call the distributor, or verify with the manufacturer. It adds time. It adds confusion.
Some pharmacies don’t even stock them because they’re harder to track. Others avoid them because insurance systems sometimes don’t recognize them as "generic" for billing purposes.

Who Benefits?
Consumers do. Authorized generics often cost 30-50% less than the brand name, even if they’re priced the same as traditional generics. That’s because the brand company doesn’t need to spend money on marketing or patent defense. They’re just selling the same product under a different name.
Insurance companies love them too. They’re cheaper than the brand, but just as reliable. No risk of unexpected side effects. No need for prior authorizations.
And for patients on long-term meds-like blood pressure pills, statins, or antidepressants-it’s peace of mind. You know you’re getting the exact same drug your doctor prescribed, just without the brand name.
What to Do If You Get One
If your pharmacy switches you to an authorized generic:
- Don’t panic. It’s safe.
- Check the active ingredient on the label. It should match your brand-name drug.
- Call your doctor if you notice a change in how you feel. (It’s rare, but if you’re sensitive to inactive ingredients, you should know.)
- Ask if the pharmacy can keep you on the authorized generic. It’s often the best value.
Many people don’t realize they’re already using authorized generics. If you take a drug like simvastatin, omeprazole, or metformin, there’s a good chance you’ve been on one for years without knowing.
The Bigger Picture
Authorized generics aren’t going away. As more drugs lose patent protection, brand companies will keep using this strategy. It’s not a loophole-it’s a legal, FDA-approved way to keep competition alive.
But it’s also a reminder that the drug market isn’t as simple as "brand = good, generic = cheap." Sometimes, the best deal is made by the same company that made the brand.
The system isn’t perfect. It’s messy. But for patients, it means more options, lower prices, and the same quality they’ve always trusted.
Are authorized generics as safe as brand-name drugs?
Yes. Authorized generics are chemically identical to the brand-name drug. They’re made in the same facility, with the same ingredients, and under the same quality controls. The FDA treats them as therapeutically equivalent. If your doctor prescribed the brand, the authorized generic is just as safe and effective.
Why don’t authorized generics show up in the FDA’s Orange Book?
Because they’re not approved as separate generic drugs. They’re sold under the original brand’s New Drug Application (NDA). The Orange Book only lists drugs approved through the Abbreviated New Drug Application (ANDA) process. Authorized generics skip that step entirely, so they’re not listed there. The FDA maintains a separate list for them.
Can I ask my pharmacist for an authorized generic?
Yes. You can ask if an authorized generic is available for your prescription. Many pharmacies stock them because they’re cost-effective and reliable. Just say, "Is there an authorized generic for this drug?" They’ll know what you mean.
Do authorized generics cost less than traditional generics?
Usually not. Authorized generics are priced similarly to traditional generics. The difference is in consistency-they’re made by the original manufacturer, so there’s less variation in quality. The real savings come compared to the brand-name version, not compared to other generics.
Are authorized generics available for all drugs?
No. Only drugs where the brand manufacturer chooses to produce one. Not all companies do. It’s a business decision, not a regulatory requirement. But for many top-selling drugs-especially those with high demand and long-term use-authorized generics are common.
9 Comments
Pat Mun
February 12 2026
So I just found out my blood pressure med is an authorized generic, and honestly? I didn’t even notice. Pill looks different, sure, but my BP’s been stable as hell for months. I used to stress about switching brands, but this? This is just the same stuff in a plain box. No drama. No side effects. Just quiet, reliable medicine.
Turns out, the brand didn’t disappear-they just stopped slapping their logo on it. Kinda like when your favorite band goes indie and stops doing interviews. You still know it’s them. Same voice. Same soul.
Neha Motiwala
February 13 2026
This is a total corporate scam. The FDA is in bed with Big Pharma. They let Pfizer make the exact same pill but call it Greenstone so they can still control the market while pretending to be ‘competitive.’ They’re not lowering prices-they’re just hiding the brand name to trick you into thinking you’re saving money. And don’t even get me started on how they manipulate the Orange Book to keep pharmacists confused. This isn’t transparency-it’s manipulation with a lab coat.
athmaja biju
February 13 2026
Indian generics are far superior. Why would anyone trust an American company to make a generic? They have patents, lawsuits, and profit motives. Real generics come from India-where they actually understand medicine, not marketing. This authorized nonsense is just another way for Wall Street to keep squeezing patients. We need real competition, not corporate theater. The FDA should ban this loophole.
Robert Petersen
February 15 2026
I love this breakdown. Seriously. I’ve been on statins for 8 years and never knew I was on an authorized generic until last year. My pharmacist had to explain it to me, and I was like-wait, so I’ve been getting the same pill the whole time? Mind blown.
It’s wild how much we assume ‘brand = better’ when the science says otherwise. The fact that the same factory, same equipment, same quality checks are used? That’s not a loophole-it’s common sense. Kudos to whoever wrote this. Clear, calm, and totally accurate.
Craig Staszak
February 16 2026
Authorized generics are the quiet MVP of the drug system. Nobody talks about them but they’re saving people thousands a year. I’ve been on one for my antidepressant for 3 years. No issues. No weird side effects. Just steady, consistent relief.
Pharmacists should be trained to flag them more clearly. It’s not rocket science. If the active ingredient matches and it’s from the original maker, it’s the same damn thing. Stop making patients second-guess their meds.
alex clo
February 16 2026
From a regulatory standpoint, the authorized generic model represents a legally sound and efficient mechanism for maintaining market competition post-patent expiry. By leveraging the original NDA, manufacturers avoid redundant clinical trials while ensuring therapeutic equivalence. This approach aligns with the FDA’s mission to promote access to safe, effective medications without compromising scientific integrity. The absence of listing in the Orange Book is a procedural artifact, not a deficiency.
Alyssa Williams
February 18 2026
So I just realized I’ve been on an authorized generic for my diabetes med for like 5 years and I didn’t even know. The pill changed color once and I thought I got the wrong bottle. My bad. But now I’m kinda proud I didn’t panic. Just checked the label. Atorvastatin. Same. Done.
PS: My pharmacist didn’t even mention it. Maybe they don’t know either. We need better labeling. Like a little sticker. ‘Made by the brand. Just no logo.’
Ojus Save
February 20 2026
wait so the same company makes the brand and the generic? thats wild. i always thought generics were from like some other country. so if i get the generic its the same as the brand? cool. i think i got one last month. didnt notice anything different. good to know.
Annie Joyce
February 20 2026
Let’s be real-authorized generics are the unsung heroes of affordable healthcare. Imagine your favorite coffee shop suddenly starts selling the exact same beans in plain brown bags for half the price. No fancy cups. No logo. Just pure, unadulterated quality. That’s what this is.
And honestly? The fact that the brand company makes it means zero risk. No weird fillers. No batch inconsistencies. Just the same pill your doctor trusted. Why does anyone care if the box says ‘Lipitor’ or ‘atorvastatin’? The drug doesn’t care. Your body doesn’t care. Only marketing does.