Switching from a brand-name NTI drug to a generic version isn’t like swapping one painkiller for another. These are medications where even tiny changes in blood levels can mean the difference between control and crisis. For drugs like warfarin, phenytoin, levothyroxine, or digoxin, a 10% shift in concentration might trigger a seizure, a stroke, or a thyroid crash. And yet, many patients are switched without understanding why-or what to watch for.
Why NTI Drugs Are Different
Narrow Therapeutic Index (NTI) drugs have a razor-thin margin between the dose that works and the dose that harms. The FDA defines them as medications where small differences in blood concentration can lead to serious treatment failure or life-threatening side effects. Unlike most drugs, where a 20% variation in absorption is acceptable, NTI drugs require near-identical delivery. The FDA’s bioequivalence standards for these drugs are stricter: instead of the usual 80%-125% range, generics must deliver between 90.00% and 111.11% of the brand’s concentration in the bloodstream. For levothyroxine, the bar is even higher-95% to 105% for total exposure.That’s why a switch isn’t just a cost-saving move. It’s a clinical event. Even though the FDA approves these generics as therapeutically equivalent, real-world data shows that some patients experience changes after switching. A 2018 study found 8-12% of well-controlled epilepsy patients had breakthrough seizures after switching from brand to generic antiepileptics. That’s not because the generic is bad-it’s because the body is sensitive. And that sensitivity needs to be acknowledged.
What Patients Are Really Worried About
When you tell a patient, “We’re switching you to a generic,” they don’t hear “same medicine.” They hear: “This is cheaper.” “Is this going to hurt me?” “Did they cut corners?” “Will I have another seizure?”Many patients have heard stories-online, from friends, or even from well-meaning but misinformed pharmacists-about people who got sick after switching. A 2017 survey showed that while 94% of pharmacists believed NTI generics were safe, only 60% routinely substituted them. That hesitation trickles down to patients. If the person prescribing it seems unsure, the patient will be too.
Patients don’t need jargon. They need reassurance rooted in facts, not assumptions. They need to know: Why this switch is happening, how it’s been proven safe, and what they need to do next.
What to Say: The Right Script
Don’t say: “This is just a generic version.”Say this instead: “I’m switching you to this generic version because it’s been tested to deliver the exact same amount of medicine into your bloodstream as the brand. The FDA requires it to be within 90% to 111% of the original-tighter than most drugs. I prescribe this same generic to my own family because I trust it works the same way.”
That’s not just information. It’s trust-building. When you say “I prescribe it to my own family,” you’re not just citing regulation-you’re showing personal confidence. That matters.
Then, immediately follow up with monitoring: “We’ll check your blood levels in 7 days to make sure everything’s stable. If you feel any new symptoms-dizziness, irregular heartbeat, confusion, or mood changes-call us right away. Don’t wait for your next appointment.”
For warfarin patients, the American Heart Association recommends INR checks within 3-5 days after the switch. For levothyroxine, TSH levels should be rechecked in 6-8 weeks. For phenytoin, serum levels should be drawn within 7-10 days. Don’t assume the patient knows this. Write it down. Give them a note.
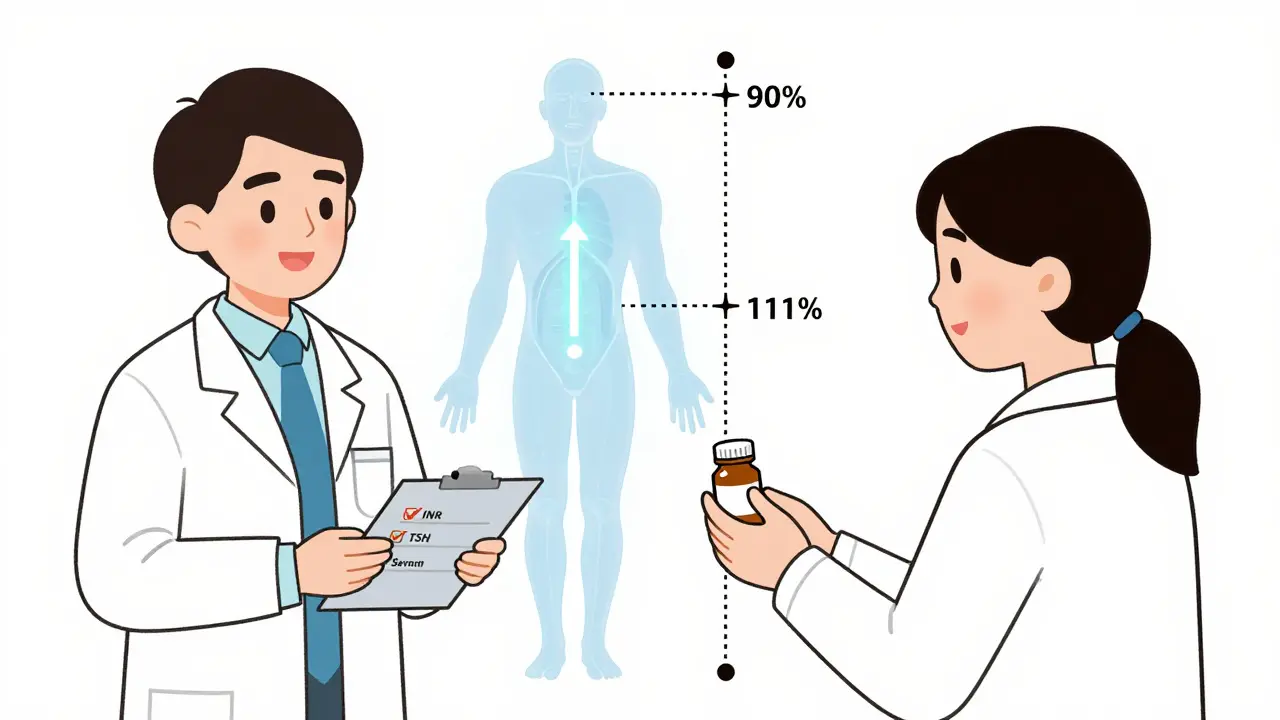
State Laws Matter-And So Does Documentation
In 27 states, there are special rules for NTI drug substitutions. Fourteen of them require written patient consent before switching. Thirteen others limit when substitution can happen. If you’re in New York, California, or Texas, you can’t just swap it on refill-you need the patient to sign off.Even if your state doesn’t require it, document everything. Write in the chart: “Patient counseled on therapeutic equivalence of generic [drug name] to brand. Advised to monitor for [symptoms]. INR/TSH/serum level to be checked in [timeframe]. Educational materials provided.”
Why? Because if something goes wrong, your documentation is your shield. But more importantly, it shows the patient you took this seriously.
Visuals Work Better Than Words
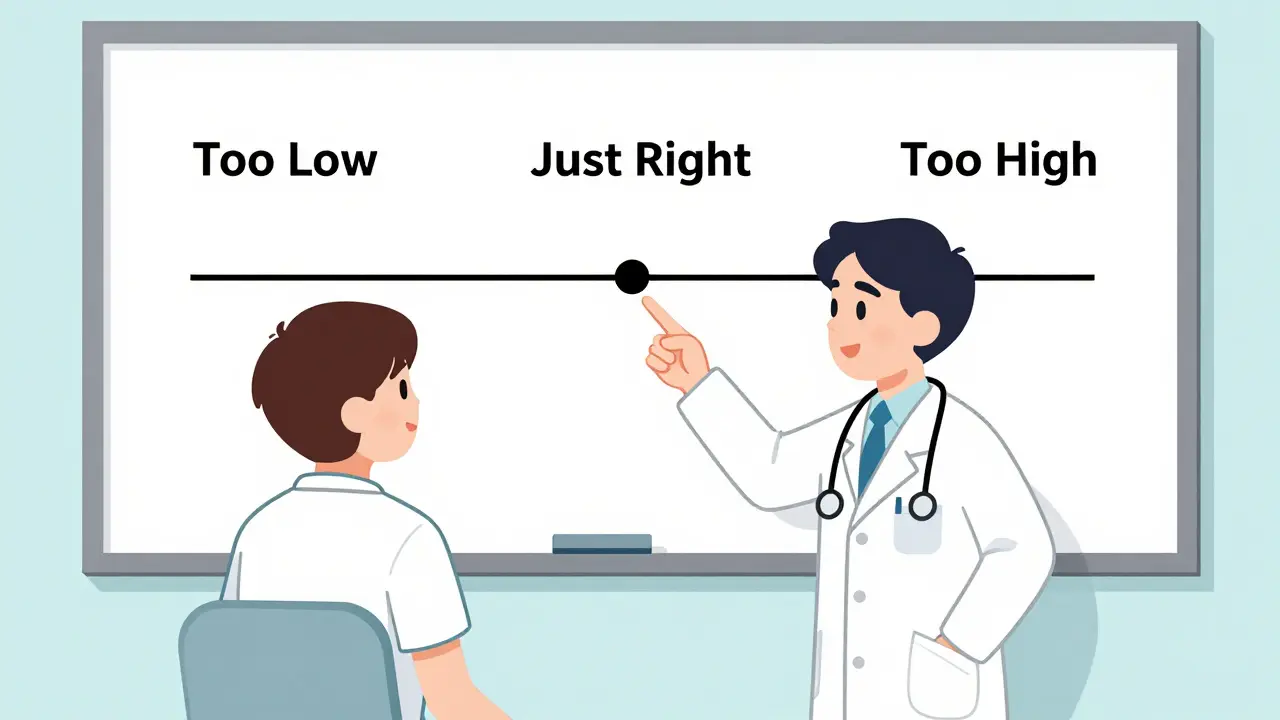
A 2023 survey of community pharmacists found that those who used visual aids-like a simple chart showing blood level ranges or a diagram of how the drug works-had 42% higher patient adherence than those who only talked.Don’t just hand them a pamphlet. Show them. Use a whiteboard. Draw a line labeled “Too Low” on the left, “Too High” on the right, and “Just Right” in the middle. Point to where their current level sits. Say: “This generic keeps you right here. We’ll check again in a week to make sure we’re still here.”
For elderly patients or those with low health literacy, this isn’t optional-it’s essential. If they don’t understand the range, they won’t recognize when something’s off.
Who Needs Extra Care?
Not all patients are the same. Certain groups are more vulnerable to changes in NTI drug levels:- Patients over 65-slower metabolism, more drug interactions
- Those with kidney or liver disease-drug clearance is altered
- People on multiple medications-especially those that affect liver enzymes (like antibiotics, antifungals, or seizure drugs)
- Patients who’ve had prior issues with switching
For these patients, consider holding off on substitution unless absolutely necessary. If you do switch, shorten the monitoring window. Check levels sooner. Talk more often.

What Not to Say
Avoid these phrases:- “It’s the same thing.” (It’s not. It’s equivalent, but not identical.)
- “The FDA says it’s fine.” (That’s not enough. Patients need context, not bureaucracy.)
- “You’ll be fine.” (Too vague. Doesn’t empower them to act.)
- “Just take it like before.” (They might not realize something’s changed.)
Instead of dismissing concern, validate it: “It’s smart to be careful with these meds. That’s why we’re checking your levels after the switch.”
The Bigger Picture: Why This Matters
NTI drugs make up just 3.2% of all generic approvals-but they account for nearly 12% of patient concerns about generics. That’s a huge gap. And while generic use for NTI drugs has been slower than for other medications, it’s growing. Sales are projected to hit $18.7 billion by 2027.But that growth won’t happen unless patients trust the process. Every time a patient has a bad experience after a switch-whether real or perceived-it fuels fear. That fear spreads. And it keeps people on expensive brand-name drugs longer than they need to be.
Good communication doesn’t just improve safety. It lowers costs, reduces hospitalizations, and builds long-term trust in the system.
What Comes Next
The FDA launched its NTI Drug Communication Initiative in 2024, offering standardized counseling checklists and patient materials in 12 languages. The American Pharmacists Association now recommends a minimum 10-minute counseling session for every NTI switch, including teach-back methods: “Tell me in your own words what you’ll watch for.”By 2025, the FDA plans to use real-world data from 12 million electronic health records to track outcomes after brand-to-generic switches. That means we’ll soon know, for the first time, exactly how many patients are affected-and why.
Until then, your words matter more than ever. You’re not just prescribing a drug. You’re managing risk, building trust, and preventing harm.
11 Comments
Kegan Powell
January 26 2026
Man i just switched my mom to generic levothyroxine last month and she’s been fine but i was terrified
they didn’t even tell her to get her levels checked
i had to call the pharmacy myself
thank god for this post
Anjula Jyala
January 27 2026
NTI drugs require bioequivalence within 90-111% per FDA but real world PK variability is often underestimated
pharmacokinetic drift even within therapeutic window can accumulate over time
especially in polypharmacy elderly with altered CYP450 activity
you’re not addressing the real issue which is lack of TDM implementation
Kirstin Santiago
January 29 2026
I love how you emphasized the "I prescribe this to my own family" line
that’s the kind of trust that saves lives
so many providers think patients just want the bottom line
but they need to feel you’re in their corner too
it’s not just clinical-it’s human
Kathy McDaniel
January 29 2026
so i just switched my dad to generic warfarin and he’s been chill but i forgot to tell him to watch for dizziness
oops
thanks for the reminder
Andrew Clausen
January 29 2026
The FDA’s 90-111% range is statistically meaningless when you consider intra-individual variability exceeds 15% in some patients
Calling generics "therapeutically equivalent" is marketing spin
the data shows increased hospitalizations post-switch in NTI drugs
and no one in this thread is addressing the real problem: the FDA’s flawed bioequivalence protocol
Paul Taylor
January 31 2026
Look i’ve been a pharmacist for 22 years and i’ve seen this play out a thousand times
you think patients care about bioequivalence ranges
they don’t
they care if they feel different
if their heart skips
if their brain feels foggy
if their mood drops
you think telling them "it’s the same" helps
it doesn’t
you need to sit down with them
draw the line
say i’ve seen this before
here’s what to look for
here’s when to call
and then you follow up
not in 6 weeks
in 7 days
because if they’re gonna crash
it’s gonna be early
and you owe them that
not a pamphlet
not a checkbox
but your time
Desaundrea Morton-Pusey
February 1 2026
Oh great so now we’re trusting generics because some doctor says he gives it to his family
what’s next
your cousin’s cousin works at Pfizer so it’s safe
the FDA is a puppet of big pharma
and now they want us to believe generics are fine
while the brand names cost 10x more
and no one asks why
this is just another way to make us sick so they can sell more drugs later
it’s not about safety
it’s about profit
Murphy Game
February 1 2026
They say the FDA approves generics
but they don’t tell you the testing is done on 24 healthy young men
not on 78-year-olds with kidney failure
not on people on 7 different meds
and when someone has a seizure after switching
they say "it’s coincidence"
but it’s not
it’s systematic
they don’t want to admit it
because then the whole generic system collapses
and the profits stop
John O'Brien
February 2 2026
bro you’re overcomplicating this
just tell the patient
"this is the same drug
same active ingredient
same FDA approval
we’re just saving you $80 a month
and we’re gonna check your numbers in a week to make sure you’re not turning into a zombie"
that’s it
no jargon
no whiteboards
just honesty and a follow-up
people get it
stop talking like a textbook
April Williams
February 3 2026
So you’re telling me we should trust a generic because some doctor says he gives it to his family
but we can’t trust the system that lets these companies make drugs in factories with no oversight
and you think writing "I prescribe this to my family" fixes the corruption
it doesn’t
it just makes you look like a naive pawn
you’re not protecting patients
you’re enabling a broken system
Harry Henderson
February 4 2026
THIS. RIGHT HERE. THIS IS THE MESSAGE WE NEED TO BE SHOUTING
not just from doctors
but from every pharmacist
every nurse
every patient advocate
you don’t just hand out a pill
you hand out peace of mind
draw that line
say i’m watching with you
check in early
listen when they say something feels off
because it’s not about the numbers
it’s about the person
and if we get this right
we don’t just save money
we save lives
and that’s worth more than any brand name